Our efforts ensured PHARMYA's compliance with operations and process management standards set by the European Medicines Agency (EMA).

Business Analysis • Standard Operating Procedures • Quality Assurance • Technical Writing • Business Process Management

The client
PHARMYA is an EU-based company, dedicated to providing Pharmacovigilance (drug safety) services. Its mission is to support the pharmaceutical industry, biotechnology companies, research centers and their service providers in their efforts to comply with international drug safety regulations.
The challenge
PHARMYA operates within a highly regulated drug safety environment where participants must maintain a required degree of process transparency and traceability at all times.
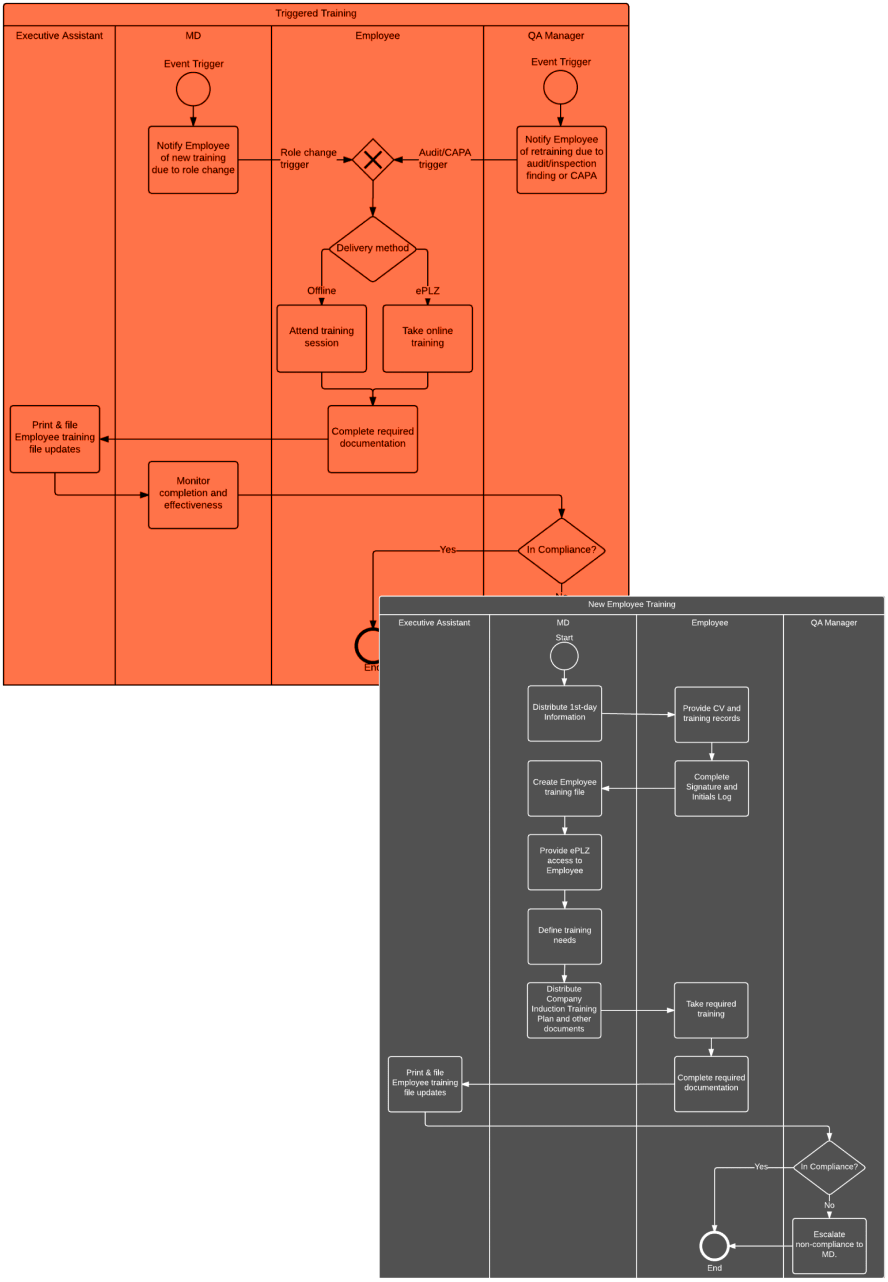
Challenges faced by PHARMYA included documentation gaps for existing processes and systems which if unresolved, would place the company at risk of non-compliance
with EMA regulations. Some of the company's Standard Operating Procedures were written at different times by different authors and lacked uniformity in terms of format and notation. Finally, two new systems being deployed by the client needed to be audited and documented in accordance with regulatory standards.
Our solution
QDC provided business process management, technical writing and quality assurance services to meet regulatory requirements and introduce a higher level of uniformity to PHARMYA's processes and documentation.

Steps to success
Results
QDC updated all existing Standard Operating Procedures to follow the same format and visual process notation, ensuring compliance with regulatory requirements and introducing a simple and easy to understand format. A repeatable Quality Assurance strategy has been established for eQMS, ePV, eLMS systems, allowing the client to eliminate known bugs and ensure a quality standard after any subsequent release. Finally, technical documentation and user guides were standardized and updated to accurately reflect the systems.


